Patient Consent
We currently offer a universal inCytes™ License Agreement form for all the users of our app. However, inCytes™ offers customizable Patient Consent for Circle Founders which allows additional documentation to be uploaded to the system in any language.
License Agreement
During the registration process, the License Agreement is presented to both the Investigator and Patient. It must be accepted by any such User prior to accessing the inCytes™ platform. This document and its acceptance are then time-stamped and stored, without access by Company personnel.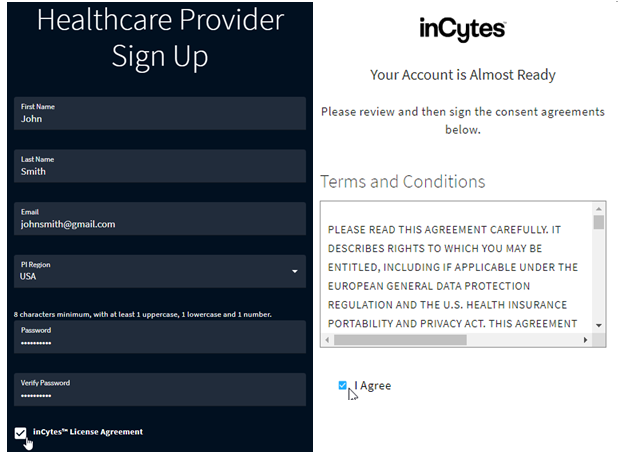
Informed Patient Consent
Consent language is typically provided by the Funder or Investigator. The patients are suggested to review and sign the consent document during their log in process. Similar to the License Agreement, we track, time-stamp and record the saved Consent, its acceptance, and the claimed identity of the accepting User, without access by Company personnel.
If the Consent is modified by the Circle, patients will be prompted to review and sign the revised document during their login process. Access to Benchmarc will only be granted once the consent is signed.
Once the patient has signed the Consent, they will be able to review each version of the accepted document from their personal portal's homepage. Investigators will not receive any notifications once the patient has signed the Consent.
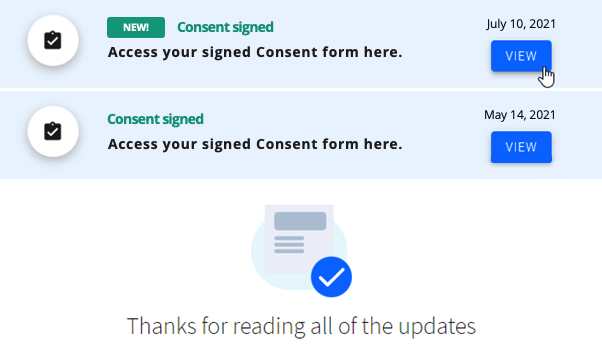
Furthermore, if a Funder or Investigator wishes to do so, they have the option to include the Consent form as a Survey. This allows the Patient to answer the questions by simply marking checkboxes. The completed survey will then be securely stored in AWS Cognito, where any modifications made to it will be logged and kept in AWS CloudTrail.
Please contact your Account Manager to learn more.
