Glossary: all terms
Definitions for terms used across inCytes platform...
A
Alerts
Text (SMS) and/or e-mail notifications sent to a user. The nature and timing of alerts are set by the user. Alerts can be set for PROMS results above or below “trigger points”, invitations to participate in a circle, overdue tasks and many other items.
B
Benchmarc™
Benchmarc™ (Patient Portal) is an interactive webpage that helps patients learn about their condition, complete surveys to report their outcomes, and view their progress over time. It serves as the central hub for all inCytes patient interactions, providing patients with compelling reasons to first enroll, and continue to complete surveys throughout recovery.
Read More about Benchmarc™
Benchmarc™ Office Brochure
Benchmarc™ provides instant access to patients own health data, allowing them to see and understand the progress they have made. With this information, patients are able to have deeper conversations with their physician, such as discussing key milestones or manage a personalized recovery plan.
To learn more, please see our downloadable Benchmarc™ Brochure.
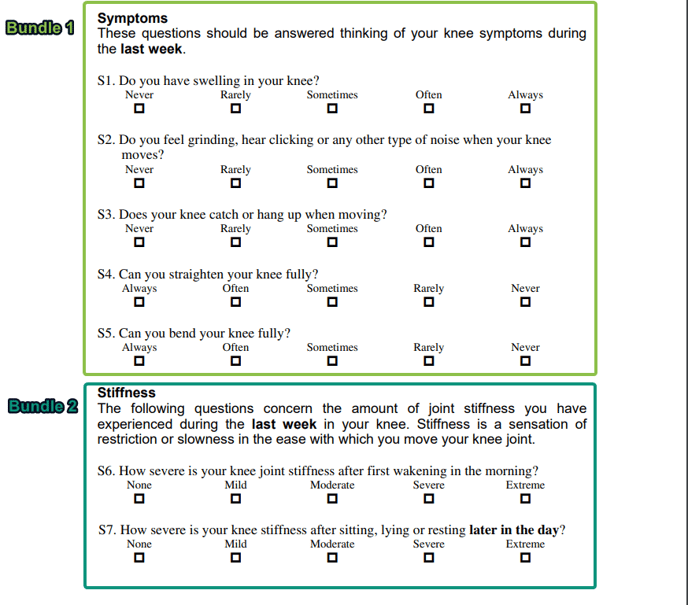
Bundles
Bundles are groups of one or more questions, whereby the answers given, whether numerical or not, are converted into numerical values. Bundles are the building blocks of Scoring Groups, and support the generation of Scores.
For example. a bundle might comprise 3 questions about the patient: (1) Pain while sitting, (2) Pain while standing and (3) Pain while walking. Each answer may have the options Severe, Moderate, Mild, which allows the patient to select the option which based matches their subjective assessment. Behind the scenes, these answers are correlated to values (Severe = 3) which then allows for the Bundle to support the algorithmic generation of a score.
C
Canonical Questions
Canonical question - it’s a unique distinguished question; one where multiple, similar, questions may be merged into.
Each question within inCytes™ has a special tag (a unique identifier) in which the system collects and aggregates the data into reports. Questions intended to collect the same data point but worded differently are recognized differently in the system – they will not be reported the same. We recommend canonical questions when possible for data collecting purposes.
Your question (as requested): What was the volume of PRP?
Canonical question (existing in the system): PRP volume obtained
Case
The application of a single observational protocol to a single patient, resulting in the collection of integrated real-world data across a clinical path.
Case Credits
Pre-paid cases available to your circle members.
Chargeable failed SMS
SMS sent by our platform but not delivered to the recipient due to the reasons on the side of the recipient as one of the following:
-
- The recipient's phone is switched off
- The number is roaming (abroad)
- The recipient opted out of SMS
- Billing issues
- Problems with the carrier network
- The recipient may be out of network
Circle
One or more users who agree to share an observational protocol and resulting observations, according to the terms established by the circle administrator.
Circle Administrator
Designated circle member who is responsible for overseeing the circle members as well as the available cases.
Circle Founder
Circle member who is responsible for creating the circle, including determining its type, name, and observational protocol. Once created, they are automatically assigned to be the circle administrator, though they can re-assign this role to another if preferred.
Circle Member
A person who shares the same observational protocol and resulting observations within one circle.
Clinical Path
The pre-treatment, peri-treatment, and post-treatment sequence in an observational protocol for one or more cases is designed to derive real-world evidence.
Circle Request Form
The Circle Request form allows a Circle Founder to formally submit a Circle request. Depending upon the options chosen, the inCytes™ team can then prepare a proposal, submit necessary documentation to begin the observational protocol process, and/or begin creating the Circle.
Please access the Circle Request Form here.
Commencement Date
Commencement date is the date which defines the actual scheduling for your Pre-Treatment surveys. It may be equal to the case creating date, but at the same time it can be as much different as it is required to be compatible with the specific country legislation in relation to the patient enrollment emails which inCytes sends. The Registration email will be sent to the patient when today is the Commencement day, not earlier.
Customization Elements
inCytes allows Users to customize a number of elements on their account to help other Users, including their Team Members, Circle Members and/or Patients, to recognize familiar branding. The User can access their various customization options through their Profile or Circle Editing screens:
1. Customizing Your Profile
2. Customize Your Circle
E
Expiration Date
I
inCytometrist
A Regen Med employee trained to assist users with observational protocols, reports, circles, and other implementations and use of the inCytes™ platform.
N
Non-PHI Circle
A Circle where the members only have access to de-identified patient information, typically aggregated across a number of cases.
O
Observation
A query plus a corresponding response, resulting in real-world data.
Observational Protocol
One or more surveys arranged along a clinical path, designed to lead to real-world evidence through the capture of real-world data.
Observational protocols, and resulting observations, may be prepared in any language without the loss of valid data aggregation and correlation.
Note: inCytes now support any non-Latin alphabet chars in Patient's and Clinician’s names, including the one from Italian, Spanish, French, etc. In the near future, we suppose to support Arabian and later Chinese alphabets.
Observational Protocol Report
Observational Protocol Request Form
To build your Observational Protocol:
- Fill in the Observational Protocol Request Form and await confirmation from your Account Manager.
- Discuss the structure and other details with your Account Manager, and our content team will build the OP on the inCytes platform based on the information obtained. The time to build your OP is dependent on the number of items requested, alongside their current presence in our library of PROMs and commonly used questions.
- After creation, you will be invited to your Circle for review and one round of revision, if necessary.
Observational Protocol Revisions
Observational Protocols have the ability to be edited at any time, but are subject to a specific process and considerations of effect upon Cases.
- Requesting the Revision
All Observational Protocol revisions must be entered upon the original Observational Protocol Request form, with changes highlighted in Yellow. This counts as 1 revision. Please request this form from your Account Manager. - Executing the Revision
Within 5 business days, and oftentimes sooner, all requested Revisions will be made live to your account, whereby you are encouraged to review the work. If a Revision was described properly in your form, and our team failed to implement, we will make the change immediately. - Revision Behavior
All revisions to Observational Protocols go into immediate effect for newly created Cases, but do not affect existing Cases. Users who wish to transfer old cases onto a new and/or Revised observational protocol are encouraged to contact your Account Manager for Custom Versioning.
Clinical realities may require quick and efficient protocol updates, even long after patients have been enrolled and reporting their outcomes. For this purpose, we offer our customers protocol versioning to seamlessly make changes to their protocols on the go, while storing preserving earlier versions for data integrity and auditing purposes.
Outliers
Survey responses or scores that fall outside of an acceptable range.
Patient ID
Patient ID - an alphanumeric code that is given to a patient while adding them to the system.
Patient De-identified Information (Non-PHI)
Observations which do not allow the identification of an individual to which such information pertains. (Compare Patient Health Information).
Patient Health Information (PHI)
Health information presented in a manner allowing the identification of an individual to which such information pertains. Governed in various jurisdictions by HIPAA, GDPR and similar regulatory regime.
Patient Report
A report presenting PHI derived from an observational protocol implemented for a specific patient. The report will include text, images, educational links, aggregated patient de-identified information for benchmarking, and other case-specific content as determined by the clinician.
PHI Circle
A circle the members of which have access to patient health information. Compare Non-PHI Circle.
PI Region
The country you choose to store your patient data (PI).
At the moment we have two options in our system:
-
- Canada
- USA
Primary Circle
The Circle which funds the case. Compare to Secondary Circle
PROMS
A Patient-Reported Outcomes Measurement survey is a standardized survey that allows patients to relay their symptoms and experience. PROMS can be repeated over time to show improvement or worsening of their condition.
Push Reminders
Small, pop-up messages sent to a user's device by a mobile app to remind of a due survey.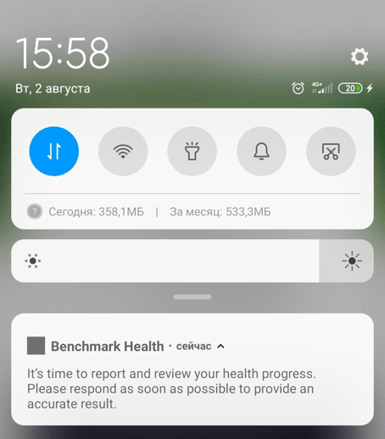
Q
Question
A question can be comprised of:
- A Prompt, which elicits an appropriate answer
- A description, which provides help in answering properly.
- An answer type, which controls how the answer is received (ie multiple choice, or numerical entry)
- Answer options, which provide pre-filled answers that the user must select (i.e. Severe vs. Mild)
- Controlled Entries, which limit the responses to acceptable entries, such as number of decimals, min-max ranges, and more.
- Settings, which might mark the question as optional or mandatory
- Localization, which makes the question available in multiple languages, but sharing the same canonical answers.
One or more questions can be placed within any Survey to be completed by the Survey's delegate.
R
Real-World Data
Data relating to patients' health status and/or the delivery of health care routinely collected from a variety of sources. An observation is designed to constitute real-world data.
Real-World Evidence
Clinical evidence about the usage and potential benefits or risks of a procedure, medical product, or another clinical element that is derived from the analysis of real-world data. An observational protocol is designed to produce real-world evidence.
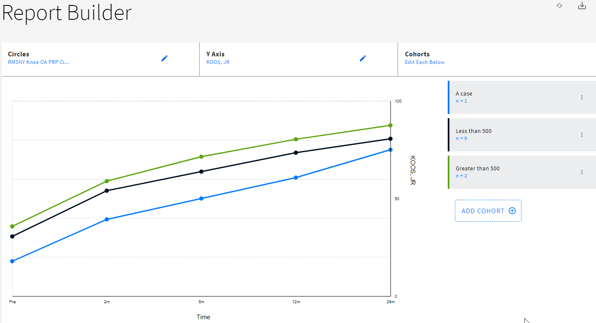
Report
The numerical and/or graphical presentation of real-world data and real-world evidence deriving from observations. The inCytes™ platform allows users to display, filter, selectively aggregate, export, and otherwise prepare reports in a manner suitable to their clinical or scientific objectives.
Response
The specific answer elicited by a query and entered onto inCytes™. Together, the query and response constitute an observation (real-world data).
S
Secondary Circle/s
Circle/s to which you may want to share the de-identified data. Compare to Primary Circle
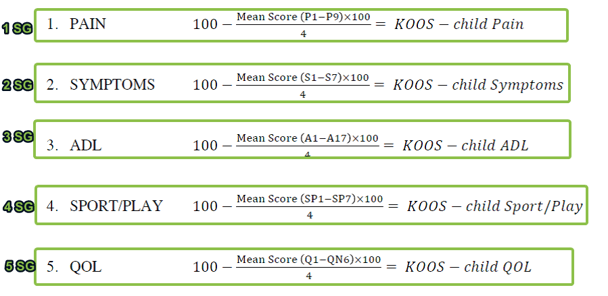
Scoring Groups
Scoring Groups apply a custom formula to one or more Bundles in order to generate a Total Score. Total scores provide the patient, clinician and/or others with quick, summarial results which are easier to interpret, aggregate and compare than the individual responses within. Scoring group examples can include BMI or other statistical formulas, Patient Reported Outcomes Measures (PROMs) or other "assessments, and also various novel, custom calculations.
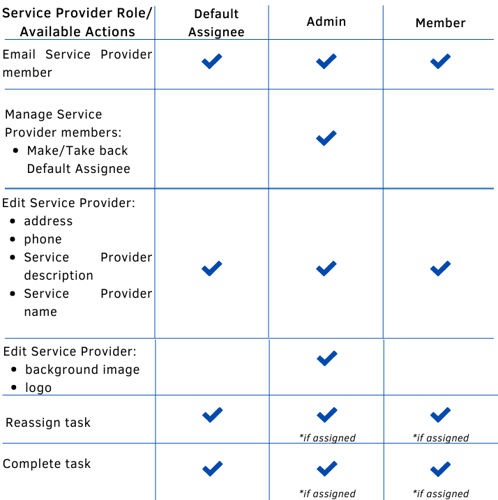
Service Provider
A third-party role for inCytes™ Circles.
This role is given to a healthcare professional that works outside of the clinic (Labs, etc.). This role helps investigators fill in the service providers' part of patient data such as the results of characterization surveys/blood tests, etc.
Subscription
Authorized access to the inCytes™ platform. Each member requires a subscription, their subscription can be self-funded or funded by a Circle Funder.
Please contact your Account Manager to purchase or cancel your subscription.
Support and Training
We provide a number of support and training services to simplify the set-up, training and ongoing usage of the inCytes™ platform. Those services, which are included and/or charged on a per hour basis, include:
- Subject to receipt of an Observational Protocol Request Form, delivery of the User's Observational Protocol within 5 business days.
- Subject to receipt of an Observational Protocol Request Form Revision, delivery of the User's revised Observational Protocol within 5 business days.
- Account implementation, which includes setting up the proper roles and customization elements for a User's account, their Team Member's accounts, Circle and Circle Member's Accounts, and more.
- Delivery of recorded training sessions and/or materials, which introduce Users to their new accounts, prepared Observational Protocols, and Instructions on Best Practices for Patient Enrollment, Survey Completion and Data Review.
- Users have full time access to the inCytes Knowledge Base, which contains hundreds of articles, images and descriptions of inCytes™ features.
Survey
One or more queries organized to elicit responses by the clinician or a patient relating to a specific portion of the clinical path. A common survey is a PROMS or a subsection of a PROMS.
T
Team
A group of healthcare workers who assist you with certain functions in your daily practice (such as case creation, patient enrollment, or circle management). Team members are granted full access to your account and can't be assigned as funders or administrators of your circle.
Team Member
A healthcare professional who assists you with certain functions in your daily practice (such as case creation, patient enrollment, or Circle management). Team members are granted full access to your account and can't be assigned as funders or administrators of your Circle.